NIFTY® Mono
by Ginekaliks laboratory
The NIFTY® Mono test, also known as the NIFTY® Monogenic test, is a specialized genetic test developed by BGI Genomics and processed in our laboratory – Ginekaliks genetic laboratory. It is part of the broader NIFTY® series of non-invasive prenatal tests (NIPT), primarily focusing on detecting monogenic disorders. These disorders are caused by mutations in a single gene, and they can lead to a wide range of genetic diseases, many of which can be serious or life–threatening.
Order now


NIFTY® by Ginekaliks laboratory
Overview of NIFTY® Mono
NIFTY® Mono test is a new and unique non-invasive single gene prenatal screening test for 202 severe congenital diseases and mental health disorders. It detects clinically significant and critical genetic conditions that until now have not been possible to determine with the classic NIPT technology, providing a more complete spectrum of potential risks after pregnancy and fetal health.
NIFTY® by Ginekaliks laboratory
Why choose NIFTY® Mono?
Ultrasound findings are not always conclusive
You would like to avoid invasive procedures for genetic analyses (ex. amniocentesis)
Family history is usually not a good indicator of probability, because these genetic conditions are caused by new ones, so-called "de novo" genetic changes (mutations)
The total frequency rate of these conditions is 1 in every 1,500 – 10,000 children
The conditions detected by this screening in 74% of cases are related to the advanced age of the father (over 35 years)
Only 5% of these diseases can be effectively treated
NIFTY® by Ginekaliks laboratory
How NIFTY® test works?
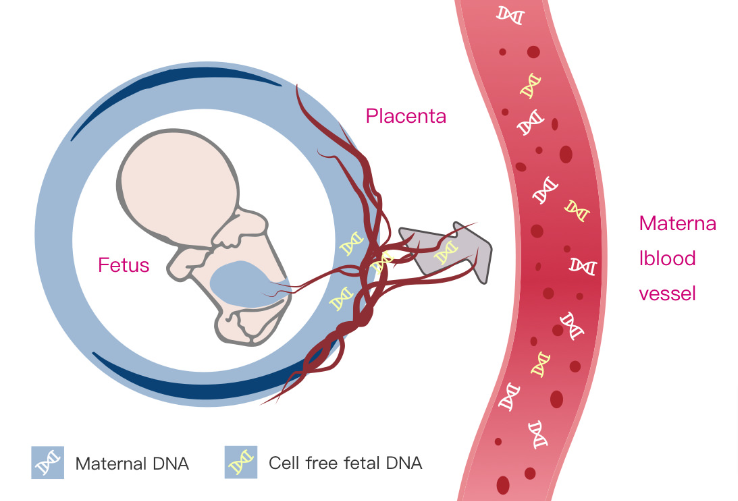
During pregnancy, fetal DNA enters into maternal bloodstream. Next-generation technology makes it possible to analyze free fetal DNA segments that are present in maternal blood in order to identify chromosomal abnormalities. NIFTY® test requires only 10 ml maternal blood sample and blood collection can be performed during the 10th pregnancy week.
1.

2.

3.

4.

5.

6.

NIFTY® by Ginekaliks laboratory
NIFTY® Mono Workflow
-

Pre-Test Counseling: Call our laboratory to consult with our geneticists free of charge and find all the answers to your questions so you can choose the best option for your baby
-

Order your prenatal test kit: Start by ordering our specialized prenatal test kit from our website. This is supported by a global leader in the logistics industry. Designed for expectant mothers, this kit includes everything needed to safely and easily collect a blood sample.
-

Visit a local laboratory for sample collection: Take the test kit to any local laboratory. A trained healthcare professional will help you gently collect a small 10ml blood sample. This process is non-invasive and 100% safe for yourself and your baby. It is quick and designed to ensure both your test convenience and confidentiality.
-

Send us a blood sample: Once your blood sample is collected, place it securely in the box provided and use the pre-addressed, postage-paid packaging to send it back to our laboratory.
-

Laboratory Analysis: Our sophisticated laboratory will analyze your sample for a comprehensive prenatal diagnosis. The fetal DNA is being isolated from the mother’s blood and is being tested with the specific targeting that cannot be detected with current NIPT technology across the whole genome with a 99% accuracy.
-

Reporting and interpretation: We will inform you on the phone and provide you via email with a detailed report that offers information for 202 monogenic abnormalities related to mutations in 155 genes (+ incidental findings) related to your baby's health and development. You will receive these results in 2 – 3 weeks quickly and safely, while having the chance to discuss the results with our specialized team and geneticists. They will help you understand your results and make sure you receive all the information, any time, 24/7 at your disposal.
Why NIFTY®?
Why you should choose NIFTY®
| NIFTY® (Non-Invasive Fetal Trisomy) | First Trimester Screening | Quad Screen | |
|---|---|---|---|
| Timing of Test | After 9 weeks of pregnancy | 11-14 weeks of pregnancy | 15-20 weeks of pregnancy |
| Detection Rate for Trisomy 21 | ~99% | ~85-90% | ~80-85% |
| Detection Rate for Trisomy 18 | ~97% | ~80% | ~70-80% |
| Detection Rate for Trisomy 13 | ~95% | ~70% | ~60-70% |
| False Positive Rate | ~0.1% | ~5% | ~5% |
| Invasive Testing Required | No | No | No |
| Risk of Miscarriage | None | None | None |
| Accuracy | Very High (based on analysis of fetal DNA) | Moderate (based on a combination of blood markers and ultrasound) | Moderate (based on multiple serum markers) |
| Follow-Up Testing | If high risk is indicated, diagnostic tests (e.g., amniocentesis or CVS) | If high risk is indicated, diagnostic tests | If high risk is indicated, diagnostic tests |
| Test Procedure | Simple blood draw from mother | Blood test and ultrasound combined | Blood test from mother |
| Turnaround Time for Results | Typically 1-2 weeks | Typically 1-2 weeks | Typically 1-2 weeks |
| Impact on Pregnancy Management | Helps inform about risk, can lead to more targeted diagnostic testing | Helps assess risk but might lead to further testing | Helps assess risk but might lead to further testing |